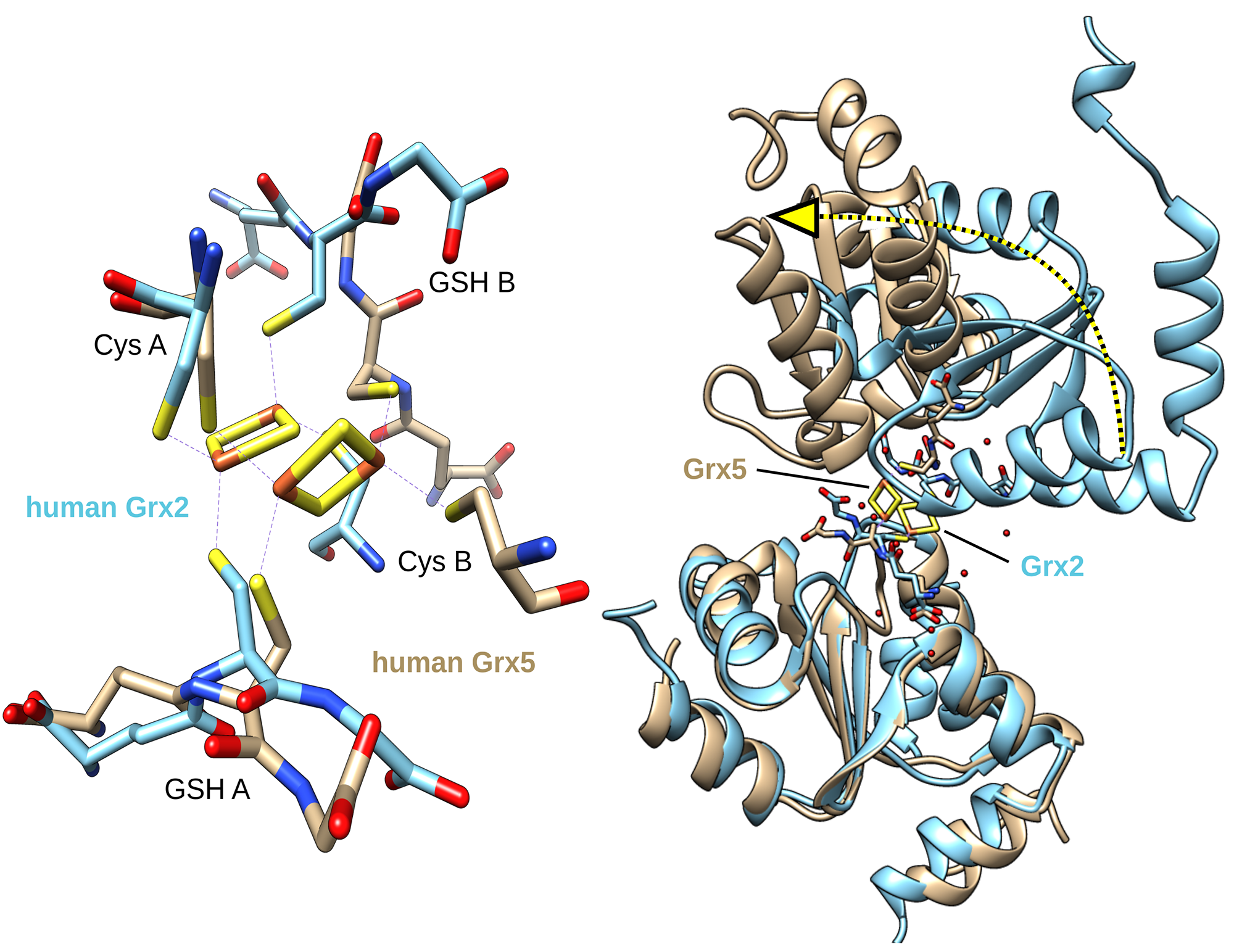
The two protein classes belonging to the glutaredoxin family are present in nearly all living organisms. Both classes are important for the energy metabolism in cells. The proteins belonging to the first class (CGFS type) are vital to the cellular iron metabolism. If this class is no longer in full working order it leads to a loss of essential iron cofactors such as haemoglobin. Haemoglobin is needed to transport oxygen. The proteins belonging to the second class (CxxC/S type) are oxidoreductases. They transfer electrons and are crucial, for example, for the development of the brain. The functions and activities of the two classes are therefore very different even though they have a very similar spatial structure and are both able to bind the cofactor glutathione.
‘Both proteins influence cell growth. If we understand more about the functions of both classes, we will gain new strategies, for example, for reducing the growth of cancer cells and we will thus be able to help people with cancer. By slowing the course of neurodegenerative diseases such as multiple sclerosis or Parkinson’s disease on the one hand, we would also be able to use the finding to encourage the growth of nerve cells on the other. The loop structure in front of the active site of the two protein classes is the decisive element,’ reports Christopher Horst Lillig from University Medicine’s Institute of Medical Biochemistry and Molecular Biology.
The international team of researchers investigated this loop structure, which is located next to the active site of the two protein classes, using targeted protein engineering from two kinds of human glutaredoxins. Using protein engineering, the scientists swapped the decisive loop structures of the two protein classes. This led to a reversal of the functions and activities of the two proteins. The identified structures are responsible for the two protein classes’ varying interaction with their joint cofactor glutathione. This in turn determines their fundamentally different functions. The gained findings shall help us not only to understand processes in cells, but also their dysfunctions.
Further Information
The term protein engineering describes the construction and production of proteins in order to change or optimise their characteristics. This can occur by means of rational design (as is the case here) or through directed evolution.
Trnka D*., Engelke A.*, Gellert M.*, Moseler A., Hossain M.F., Lindenberg T., Pedroletti L., Odermatt B., de Souza J., Bronowska A., Dick T., Mühlenhoff U., Meyer A., Berndt C., Lillig C.H. (2020): ‘Molecular basis for the distinct functions of redox-active and FeS-transfering glutaredoxins’ in: Nature Communications.DOI: 10.1038/s41467-020-17323-0
* These authors contributed equally to the publication.
Funding: Deutsche Forschungsgemeinschaft (DFG) Grant numbers: BE 3259/5-1, BE 3259/5-2, BE 3259/6-1, DI 731/3-2, GRK1947-A1, LI 984/4-1, ME 1567/9-1, ME 1567/9-2, ME 1567/13-1.
Contact at University Medicine Greifswald
PD Dr. Dr. Christopher Horst Lillig
Institute of Medical Biochemistry and Molecular Biology
Ferdinand-Sauerbruch-Straße, 17475 Greifswald
Tel.: +49 3834 86 5431 (or 86 5407)
lilligchristopherhorstmed.uni-greifswaldde
presselilligde
http://www.lillig.de
Twitter: @RCOF8